C3h8 Has a Boiling Point Than C2h6.
The smallest CH4 likely has the weakest intermolecular forces. C3H8g 5 O2g 3 CO2g 4 H2Ol ΔH -2220 kJ How much heat is evolved.
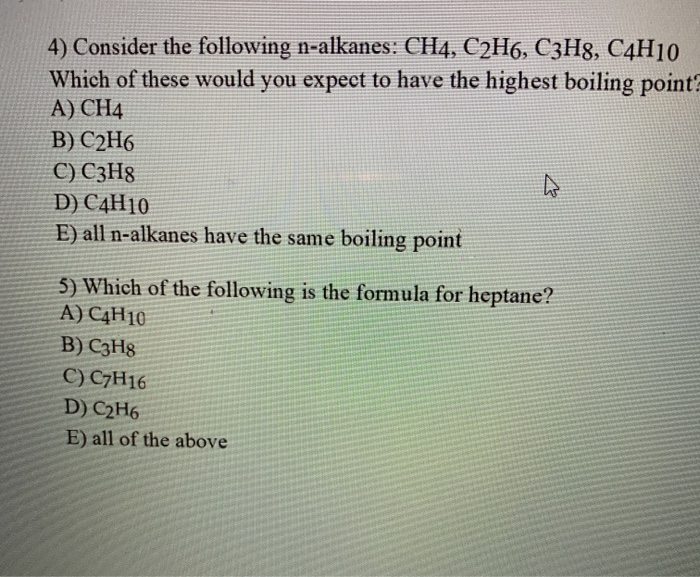
Solved 4 Consider The Following N Alkanes Ch4 C2h6 C3h8 Chegg Com
Methane is thus a polar molecule.
. CH4 C2H6 C3H8 CH3COOH 2. In cases like this the heavier molecules which has stronger dispersion forces will have the higher boiling point. Butane C4H10 -05 -135 gas.
The particles are longer therefore established larger short-term dipoles and can lie better together than the much shorter fatter 2-methylpropane. C2H6 or C3H8 3-C3H8 has higher BP-Since it has greater Mr. Why does C4H10 have a higher boiling point than C2H6.
A HF has a higher boiling point than HBr. These are attractions between the molecules of a substance such as dispersion forces dipole-dipole interactions and hydrogen bonding. Ethane C2H6 -89 -172 gas.
C2H6 C3H8 Why does butane have a high boiling point. Of 118C Substance C with a bp. A C2H6 B C3H8 C C5H12.
Boiling point of 198C Substance B with a bp. CH3COOH Compounds with stronger intermolecular forces will have higher boiling points ion-ion hydrogen bonding dipole-dipole london dispersion. B H2O2 has a higher melting point than C3H8.
Its boiling point is higher than the C2H6 because although it has no hydrogen bonds the molecule is polar due to the difference in electronegativity dipoles-dipoles. Methoxymethane CH3OCH3 and ethanol C2H5OH have the same relative molecular mass. All boiling points are normal boiling points Group of answer choices Substance A with a bp.
Butane has a higher boiling point due to the fact that the dispersion forces are higher. Of 100C Substance D with a bp. - C3H8 - CH3COOH - CH4 - C2H6.
Propane has the chemical formula. -42C and a melting point of. The stronger the IMFs are the harder it is for the molecules to loosen and thus transition state.
There are only dispersion forces and these are stronger in the heavier C4H10 molecule. Alkane Formula Boiling point C Melting point C Density gcm3 at 20C Methane CH4 -162 -183 gas. CH3COOH is the only one that is capable of hydrogen bonding so it will have the highest boiling point.
This makes its molecules harder to separate so it takes more energy thus the higher boiling point. Using data the boiling point constants given in the test book calculate chemistry The complete combustion of propane C3H8g is represented by the equation. Which of the following is expected to have the lowest normal boiling point.
Meltingboiling points are measures of the thermal energy required to break the intermolecular forces IMFs in a substance. C2H6 has the higher vapor pressure. Which of the following is expected to have the highest normal boiling point.
Determine whether or not it is necessary to break bonds or to simply overcome inter- molecular forces in each of the following processes. The C-O bond dipoles reinforce each other so the molecule has a dipole moment. Which of these compounds has highest boiling point c3h8 c8h18.
Finally the C-H bonds in methane are nonpolar so the molecule is also nonpolar. D KOH has a higher boiling point than CH3OH. 1238 Which of each pair has the higher vapor pressure a C2H6 or C4H10.
None of these have hydrogen bonding. Pentane C5H12 36 -130 0626. Br2 F2 I2 Cl2 Answer Higher boiling points will correspond to stronger intermolecular forces.
Ethane having a higher boiling point than methane is because molecules of ethane C2H6 having more Van der Waals forces intermolecular forces with neighboring molecules than for methane CH4 due to the greater number of atoms present in molecules of ethane compared to methane. Boiling point of C3H8 is -42 degree celsius. C2H6 will only have Van der Waals forces and as the molar masses of all the compounds is very similar the strength of the Van der Waals will also be very similar.
Of 783C Substance E with a bp. List the following molecules in order of increasing boiling point. A C2H6 B C3H8 C C5H12 D C4H10 E CH4 D C4H10 Only London forces so molecule with the most electrons will be highest boiling.
Which of the following will have the highest boiling point. Dipole-dipole forces are not as strong as hydrogen bonds so dimethyl ether has a lower boiling point than methanol does. C3H8 a boiling point of.
Propane C3H8 -42 -188 gas. Explain why methoxymethane has a much lower boiling point than ethanol 3-Methoxymethane is very weakly polar-Weak van der waal forces exist between methoxymethane molecules. Go through the list above.
C GeH4 has a higher boiling point than SiH4. Helpful 0Not Helpful 0 Add a Comment. Methanol has a higher boiling point than methane because it has stronger intermolecular forces IMFs which are attractions between individual molecules.

Structural Formula Molecular Teaching
Which Of These Compounds Has The Highest Boiling Point C3h8 C8h18 C12h26 Or C16h34 Quora
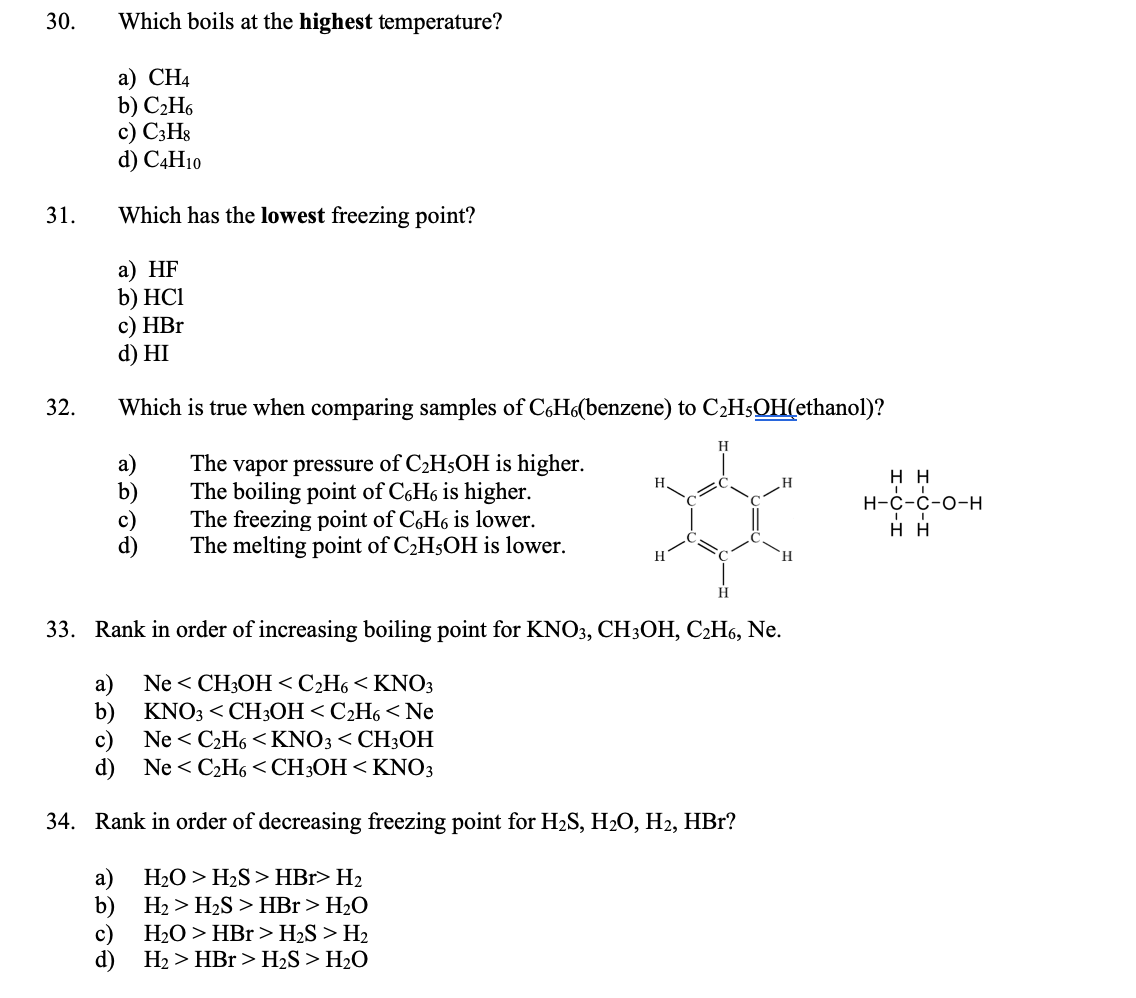
Solved Which Boils At The Highest Temperature A Ch4 B Chegg Com
Comments
Post a Comment